
/Atom-58e4f7173df78c51626061cc.jpg)
An ion is nothing more than an electrically charged atom. If you need to know how the electrons are arranged around an atom, take a look at the ' How do I read an electron configuration table?' page.Īn atom can gain or lose electrons, becoming what is known as an ion. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons.Įlectrons are arranged around atoms in a special way. Atoms must have equal numbers of protons and electrons. That means that there must be a balance between the positively charged protons and the negatively charged electrons. For example, removing one proton from an atom of krypton creates an atom of bromine.īy definition, atoms have no overall electrical charge. Adding or removing protons from the nucleus of an atom creates a different element.
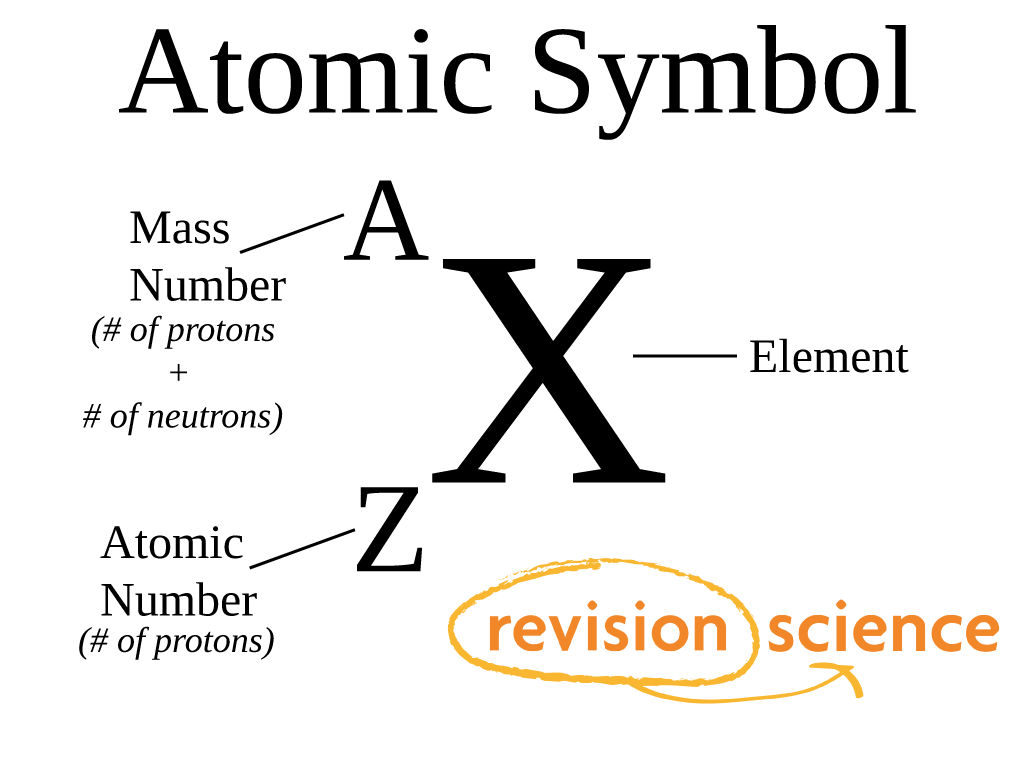
If an atom doesn't have 36 protons, it can't be an atom of krypton. The interesting thing here is that every atom of krypton contains 36 protons. This tells us that an atom of krypton has 36 protons in its nucleus. In our example, krypton's atomic number is 36. The atomic number is the number of protons in an atom of an element. The atomic number is the number located in the upper left corner and the atomic weight is the number located on the bottom, as in this example for krypton: Use the Table of Elements to find your element's atomic number and atomic weight. If it makes things easier, you can select your element from an alphabetical listing. Go to the Periodic Table of Elements and click on your element. The first thing you will need to do is find some information about your element.

To find the number of protons, electrons and neutrons in an atom, just follow these easy steps: How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc.?


 0 kommentar(er)
0 kommentar(er)
